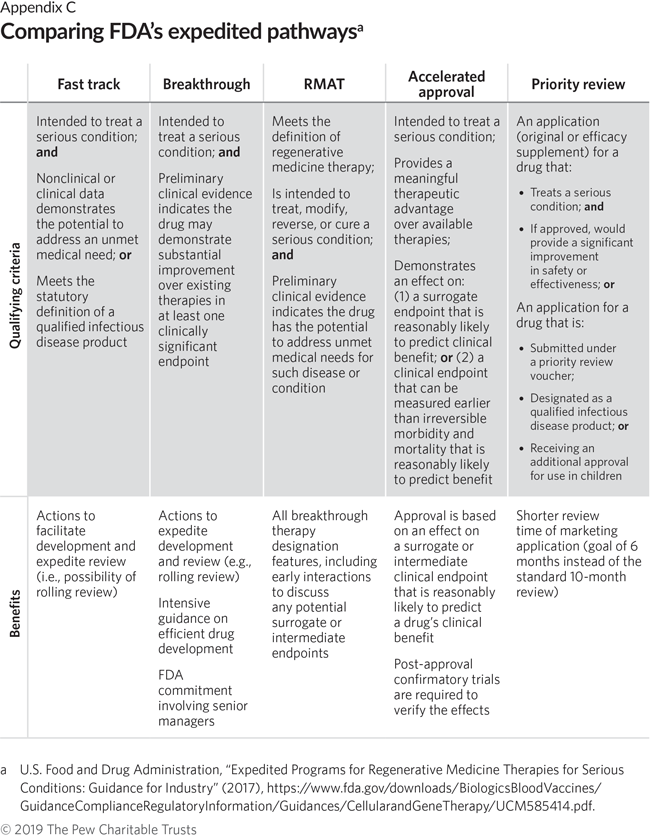
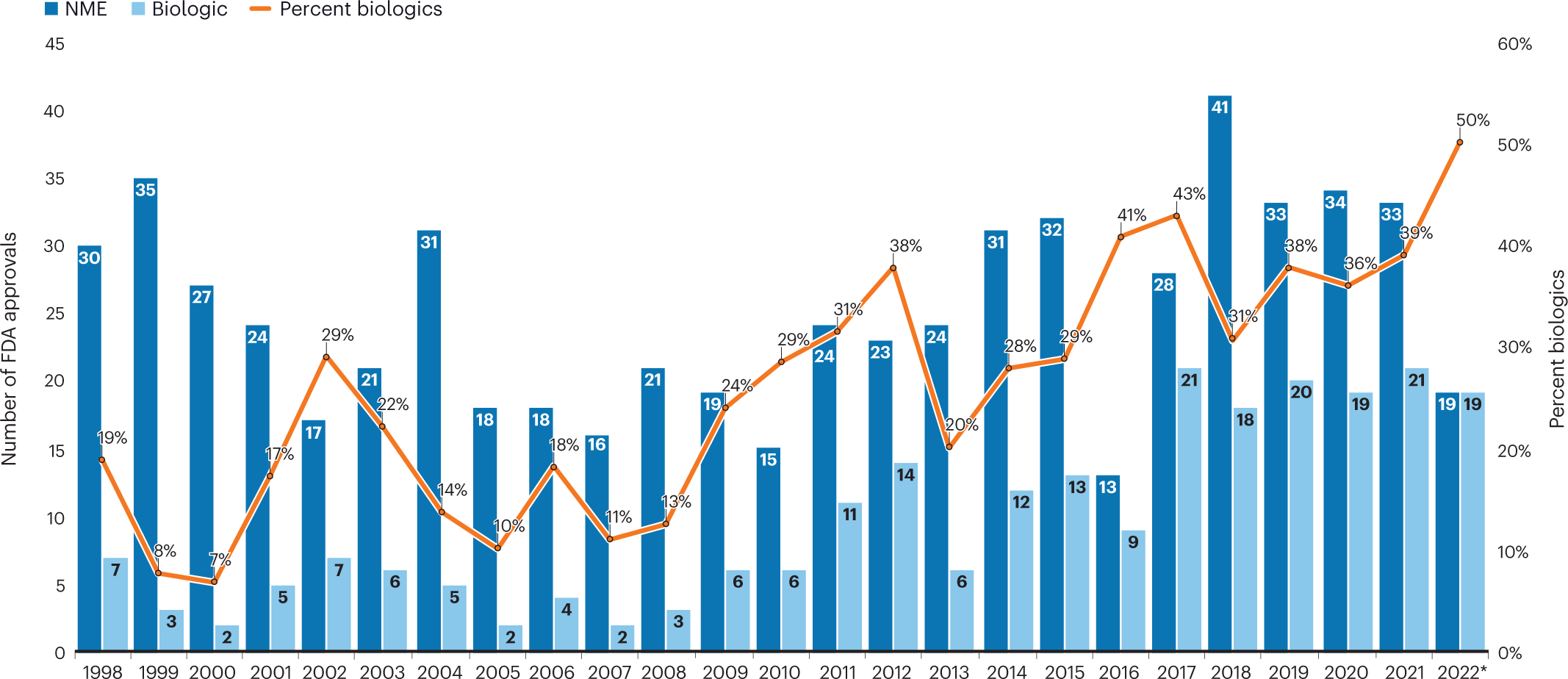
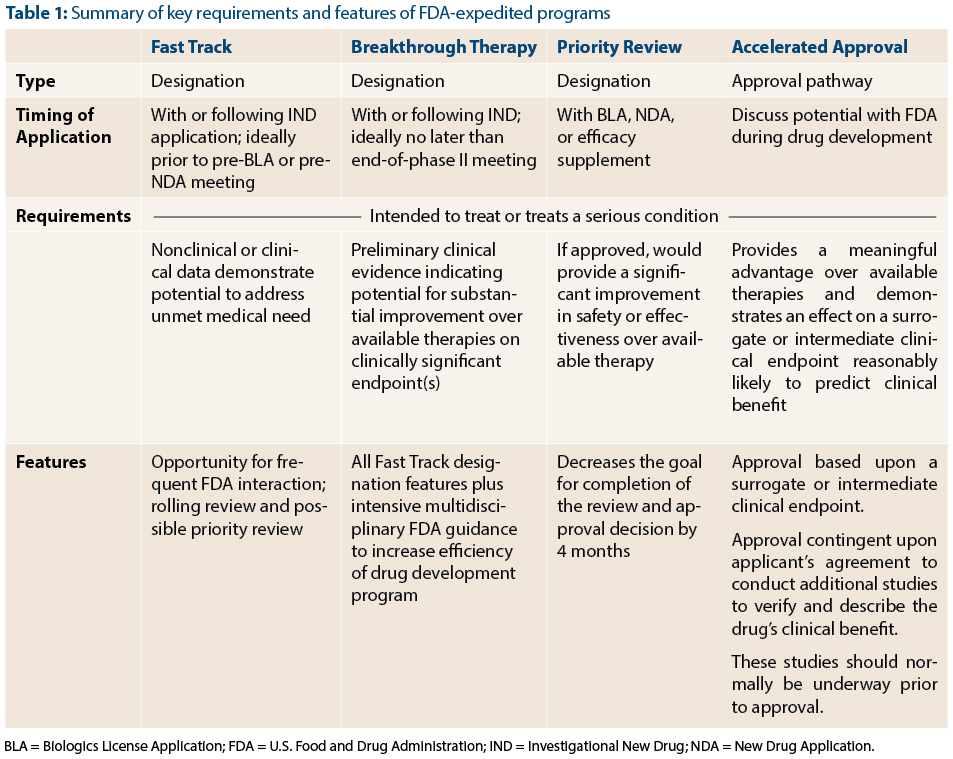
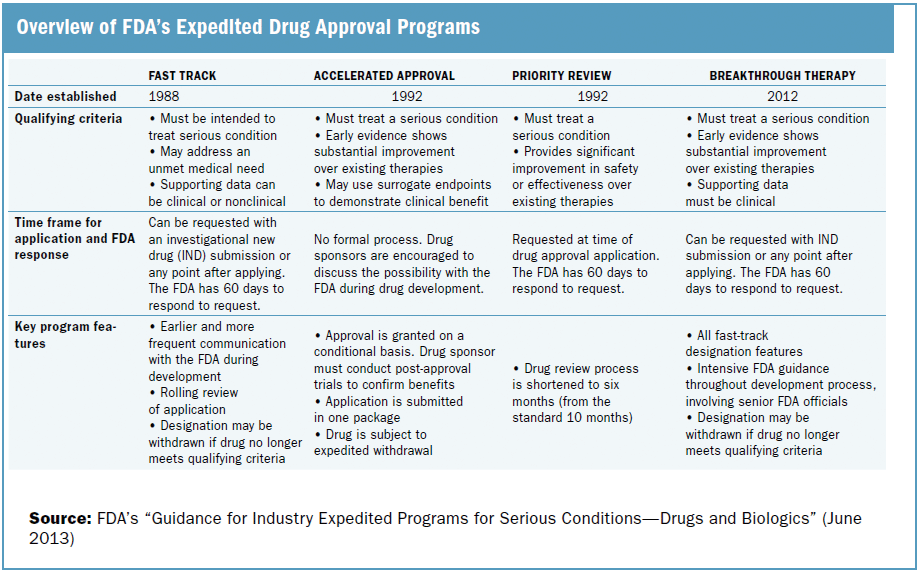
CytoDyn Inc. submits the first of three main sections of its HIV Biologics License Application to FDA under rolling review

CorMedix eyes US FDA priority review for bloodstream infection drug Defencath | S&P Global Market Intelligence

Analysis of the Real-Time Oncology Review (RTOR) Pilot Program for Approvals of New Molecular Entities | SpringerLink
Development and Regulation of Medical Countermeasures for COVID-19 (Vaccines, Diagnostics, and Treatments): Frequently Asked Questions - EveryCRSReport.com