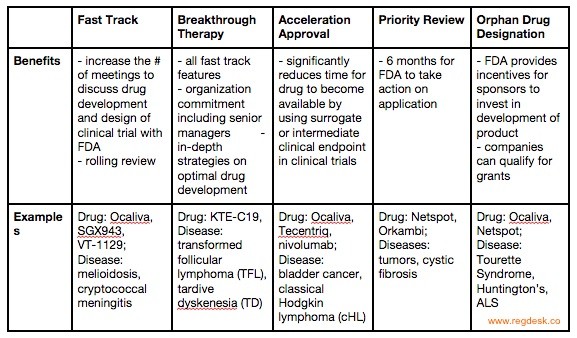
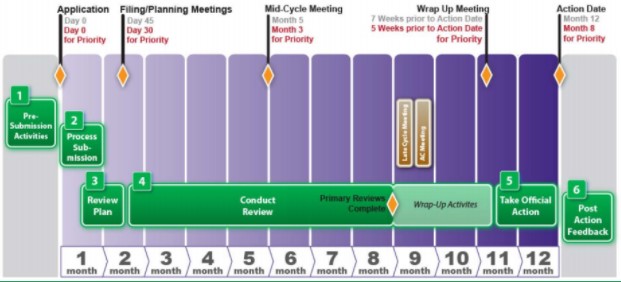
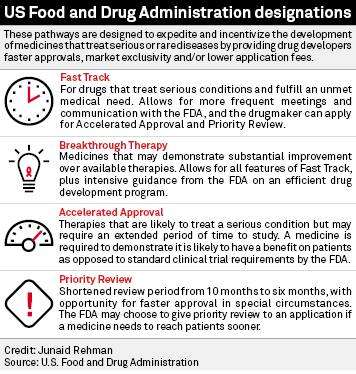
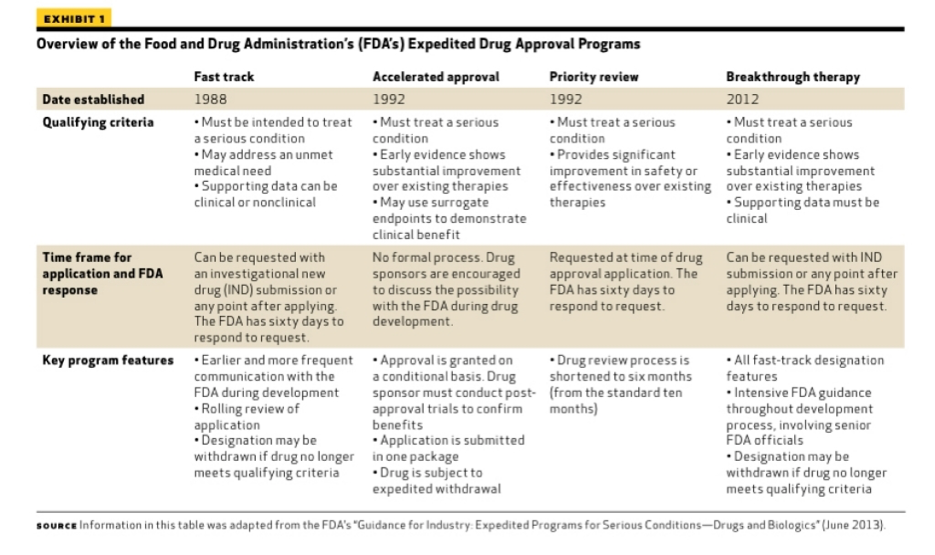
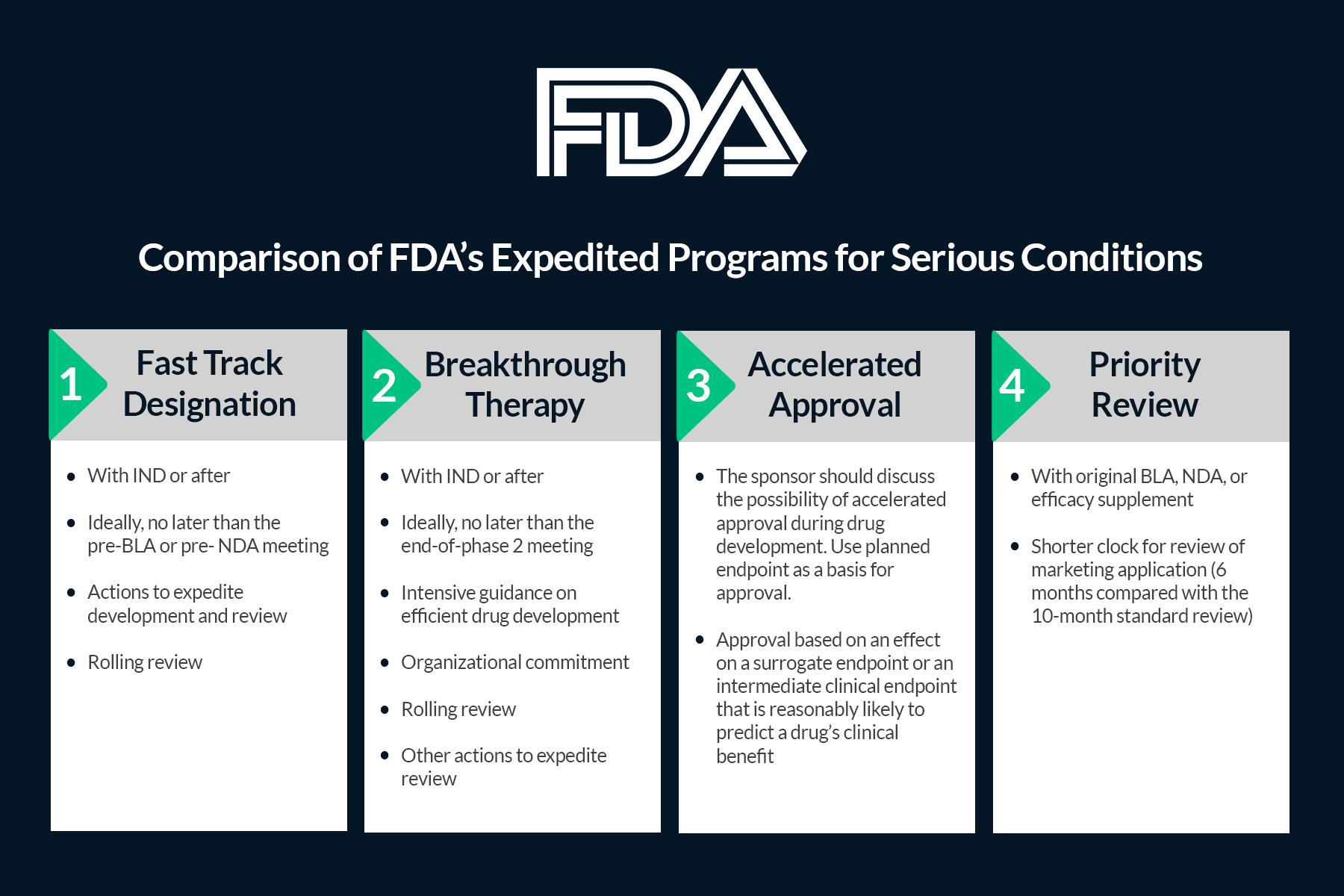
Vertex/CRISPR to begin rolling review in US for gene-edited therapy; get FDA designations | Seeking Alpha

CytoDyn Inc. submits the first of three main sections of its HIV Biologics License Application to FDA under rolling review

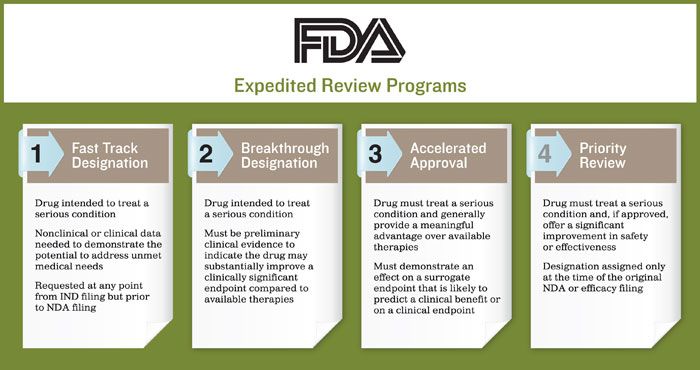
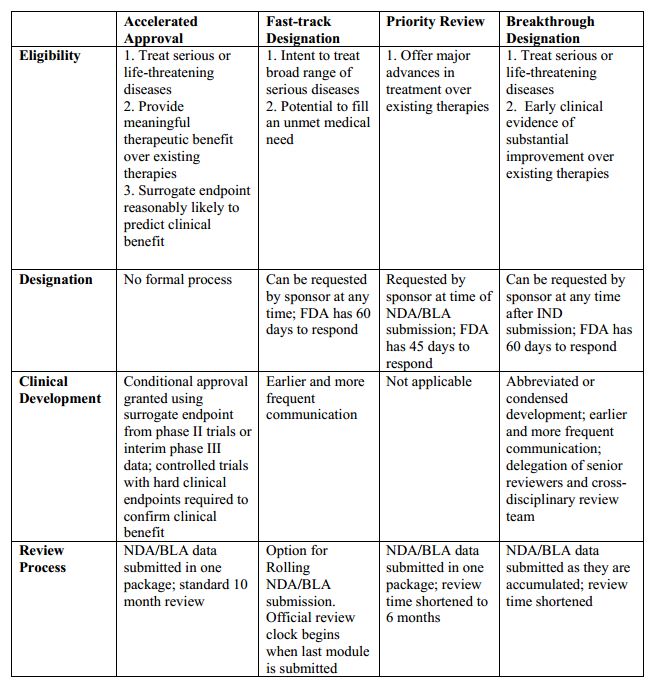
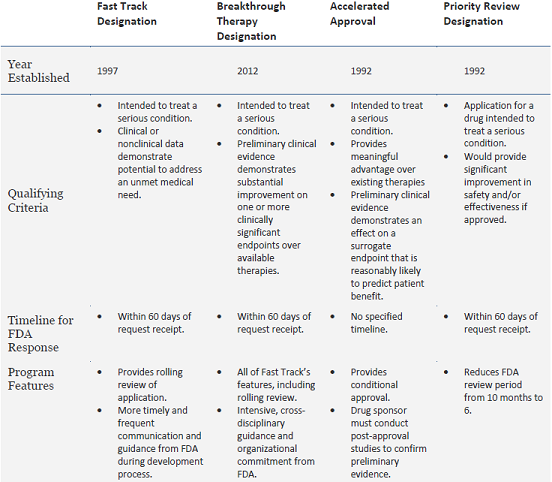
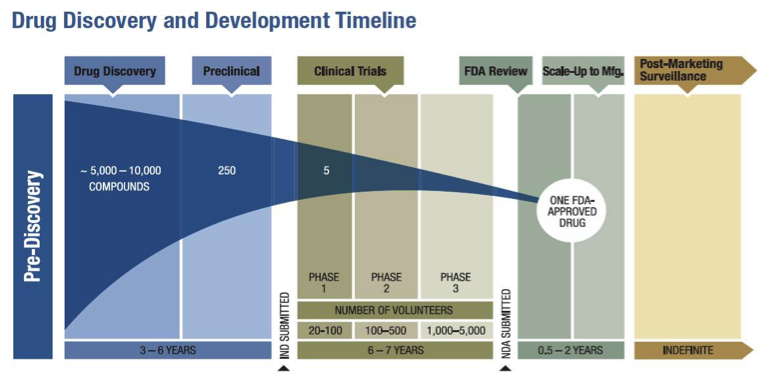
Update to Drugs, Devices, and the FDA: How Recent Legislative Changes Have Impacted Approval of New Therapies - ScienceDirect

A detailed analysis of expedited regulatory review time of marketing authorization applications for new anticancer drugs in the US and EU - da Costa Gonçalves - 2022 - Clinical and Translational Science - Wiley Online Library

Analysis of the Real-Time Oncology Review (RTOR) Pilot Program for Approvals of New Molecular Entities | SpringerLink
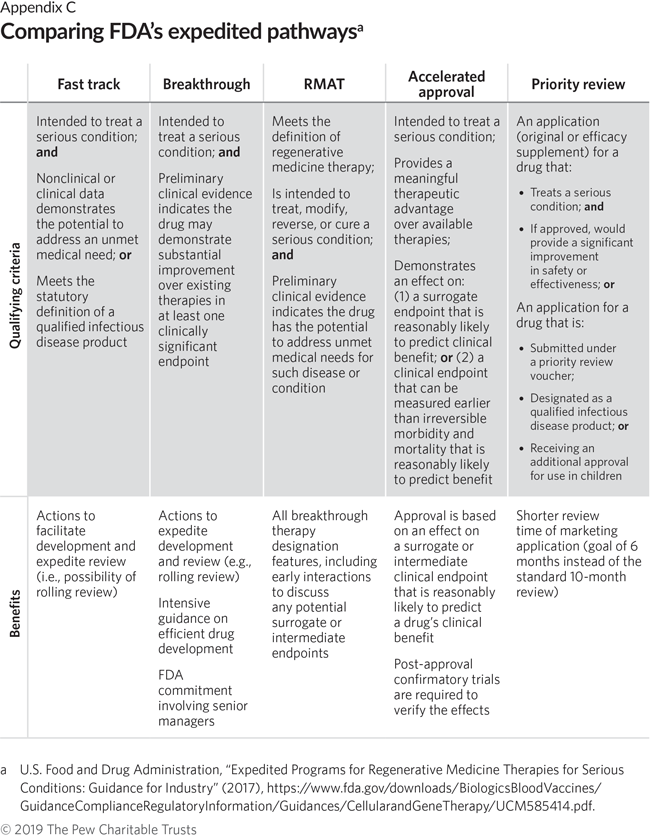
FDA's Framework for Regulating Regenerative Medicine Will Improve Oversight | The Pew Charitable Trusts